Steel
Steel is one of the pillars of modern civilization but its production emits far more CO2 than the aviation industry. Material substitutes for steel simply don’t exist on the kind of scale needed. Reducing the carbon impact of steel production faces major technical and economic challenges.
Materials don’t get as much attention as fossil fuels in climate change discussions, but materials are, if you’ll pardon the expression, foundational to the challenges we face. Steel is one of those critical materials, called by Vaclav Smil one of the four pillars of modern civilization (the other three are ammonia, concrete, and plastic) in his brilliant 2023 book “How the World Really Works”.1 Steel production generates a lot of CO2, with direct and indirect emissions accounting for 9-11% of the world’s total.2 To put that in context, those emissions are nearly 3-4 times aviation’s 2.5% share.3
We are going to take an extended, multi-article look at the carbon emitted by steel production, what is being done to reduce it, and what might be done in future.
First, we’ll look at why steel is so important and why we are so dependent on it. To do that, it will be helpful to review how we got here.
The pre-history and early history periods of civilization were dominated, even defined, by the technologies available to humans. The periods are often more narrowly defined by the tools available, or even more specifically, the materials those tools are made of. We are all familiar with the Three-Age system for classifying those tool materials–Stone, Bronze and Iron Ages–a concept first introduced by Danish archaeologist Christian Thomsen in 1836.4 These were no doubt preceded by a Wood Age, but wood doesn’t survive well in the archaeological record, so that age was overlooked by Thomsen.
The three most desirable properties of any tool material are that it be strong, stiff, and light. With most materials, you get two out of three, at best. The material should also be relatively easily worked, and if it doesn’t fracture in use too readily, that’s a bonus.
Stone is stiff and strong but heavy and prone to fracture. It also has the virtue of being found nearly everywhere lying about on the surface, though the most suitable stone was a scarce commodity.
Bronze (an alloy of copper plus tin, originally) is lighter and stiffer, but not as strong, and is also prone to fracture. Using bronze also ushered in two other technologies that are still crucial today - smelting (getting the pure metal out of the ore it is found in), and casting (making useful shapes by pouring molten metal into moulds).
Pure iron is strong and stiff but also heavy, and less prone to fracture.
We could now be said to be in the Steel Age. What is steel, exactly?
Steel is comprised mostly of iron. Iron is an element that sits in the fourth row of the periodic table with an atomic number of 26, and is the fourth most abundant material in the mass of our planet. The Earth’s core is comprised mostly of iron, where the fluid circulation of its molten outer layer generates the Earth’s magnetic field. There is much less iron in the Earth’s crust, where it makes up only about 5% of its mass.
The earliest use of iron seems to have been making tools from iron-rich meteorites, but these were relatively scarce.5 Most iron in the Earth’s crust occurs in the form of ore composed of iron oxides (iron plus oxygen, and sometimes hydrogen) of one sort or another, which can be thought of as a kind of exotic rust. The iron is extracted from the ore by smelting, which means separating the oxygen in the oxides from the iron by heat, sometime with additional reactants.
From ancient times, iron was smelted by two main processes. In a type of kiln known as a bloomer, charcoal was used to make a fire that reduced the oxygen in the ore without melting the ore. This process yielded wrought iron, which has almost no carbon–typically less than 0.1% by weight. Wrought iron isn’t terribly strong but, when heated, is malleable and easily worked by a blacksmith.
Get the fire hot enough to melt the iron by forcing air into the carbon-fueled fire, and the result is pig iron, which has a lot of carbon in it, relatively speaking (4-5% by weight). That much carbon adds a lot of strength but also makes the iron very brittle. Pig iron is usually an intermediate step on the way to making cast iron, which has a lower carbon content (2-4%) but is not so brittle. Cast iron was a favourite structural material starting in the late 18th century. The world’s first cast iron bridge was built in 1779 over the River Severn in the UK and is still standing. Cast iron is still important today, with a typical application being internal combustion engine blocks.
Steel occupies a sweet spot between wrought iron and pig iron in terms of carbon content (0.15 - 2.0%). Steel is stronger and stiffer than wrought iron, and more flexible and less brittle than the cast iron. Steel is also generally alloyed with other elements to produce desirable properties such as additional strength, corrosion resistance, and workability.6
Iron was produced in larger quantities in various ways during the Industrial Revolution, including hand “puddling” (stirring the molten iron by hand) in a reverberatory furnace to reduce the carbon content.7 It was highly-skilled work–a Puddler was the highest-paid craftsman in the 1830s (also among the shortest lived)–but production volumes were limited.
Mass iron and steel production was really only made possible by the invention of the open hearth blast furnace by Henry Bessemer in the 1850s. The process uses coal or coking coal (coal with the volatiles driven off) mixed in with the ore to melt it, by blowing air through the molten mass to burn the carbon in the coal. The burning carbon adds more heat to the process, which also burns out the oxygen from the iron oxide ore, leaving the iron and not much carbon. When enough carbon is removed, the low-carbon iron becomes steel.
A resulting unwanted byproduct of producing steel with a Bessemer blast furnace is that large amounts of CO2 are emitted from the burnt coal needed to drive the process.
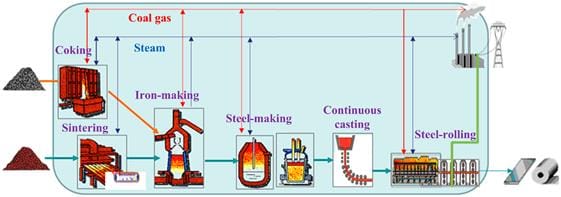
The Bessemer process ushered in what we could rightly call the Steel Age, because of its rapid adoption. Steel is found everywhere now because it is an extremely versatile material due to its combination of hardness, tensile strength, formability, adaptability (through changing its chemical composition), heat resistance, and relatively low cost. When scrapped, steel is nearly infinitely recyclable via re-smelting. For these and other reasons, steel enables modern civilization.
Tall skyscraper buildings would not be possible without steel frames to hold them up, or indirectly as reinforcing bar for concrete. Most of the structure of ships, cargo railroad rolling stock, rails, and road transport is made of steel.8 Steel is rolled into beams and sheets, cast and stamped into complex shapes, and drawn into wires to make cables. With the addition of a lot of chromium, steel becomes very corrosion-resistant and is then called “stainless steel”. Stainless steel can be found in everything from kitchen utensils, to cladding on the world’s largest rocket ship, SpaceX’s Starship.
The majority of crude steel manufactured in the world today is still a product of modern variations on the Bessemer process, albeit with more steps and more complexity, particularly the addition of two technologies. The first is called a Basic Oxygen Furnace (BOF), a more efficient way of oxidizing the pig iron or scrap iron. The second is the Electric Arc Furnace (EAF), which is a technology that uses scrap iron and steel instead of basic iron ore.9 However, the basic blast furnace ore-to-iron process still requires a lot of coal or coke, which is largely why steel has such a large carbon footprint.
The world produces about 1,800-1,900 million tonnes of steel per year, a number which has been reasonably stable for the past eight years. To put that in context, that is 219 kg (483 lbs) per person per year and is ten times the amount produced in 1950. About 71% of the steel is produced by the BOF process, with about 29% produced by the EAF process. Each tonne of steel requires, on average, 20.99 GJ of energy to produce. Production of crude steel emits 1.91 tonnes of CO2 per tonne of steel on average, but the BOF process emits much more carbon, at 2.33 tonnes per tonne, than does the EAF process, at 0.89 tonnes per tonne.10 For perspective, 2.33 tonnes of CO2 is roughly equivalent to the CO2 from combusting 992 L (262 US gal) of gasoline.11
In a BOF process, as you would expect, the blast furnace and the coking coal plant are responsible for over 80% of the CO2 emitted:12
|
Process Step |
Fraction of
CO2 Emitted |
|
Coke Plant |
32% |
|
Sinter Plant |
8% |
|
Pellet Plant |
2% |
|
Blast Furnace |
50% |
|
BOF Plant |
7% |
Steel production is distributed surprisingly widely across the world, but not evenly. Steel is produced in 92 countries, but most contribute rather small amounts to the world total. China produces over half (53.3%) of the world’s steel. The top dozen producing countries together account for nearly 87% of the total. The remaining countries each produce one percent or less.13
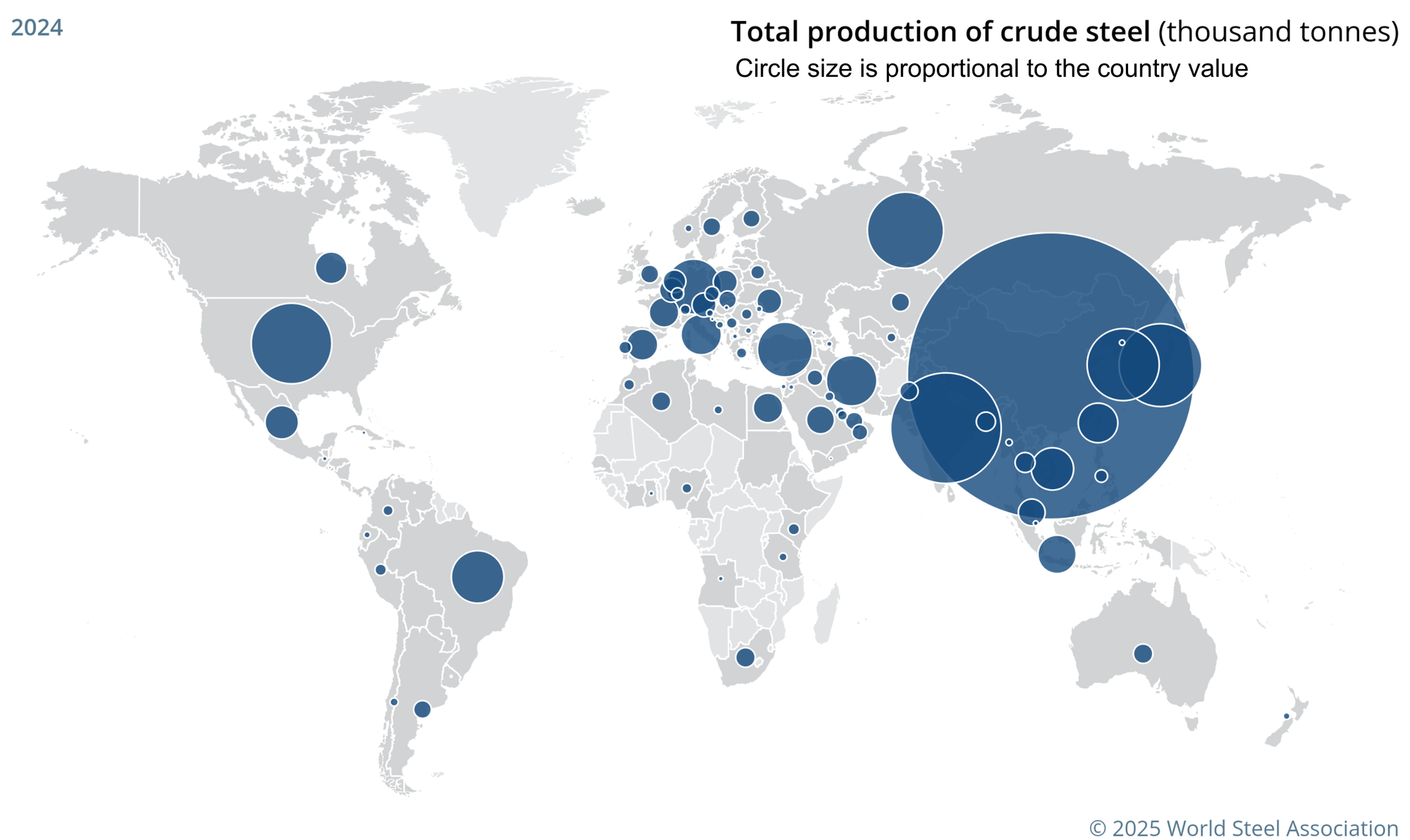
Steel isn’t about to lose its place as one of the pillars of modern civilization any time soon. Substitutes simply don’t exist on the kind of scale needed.
As we see it, reducing the carbon impact from steel production faces two fundamental challenges:
- The inherent requirement for high-carbon fuels in the Bessemer process, together with its high energy requirements. These are primarily technical challenges, necessitating the development of innovative low-carbon and lower-energy methods to de-oxidize iron ores and scrap steel.
- Economic and market challenges. The global steel market faces overcapacity, volatile raw material and energy costs, supply chain instability, along with recently increased tariffs, and other protectionist measures.14 All these factors massively increase market uncertainty, which will make it difficult for steel producers to commit to the new capital investments that will be needed to adopt the new low-carbon technologies and processes.
The next stories in the series will cover some of the work being done to develop alternative processes to extract the iron from the iron ore and scrap steel, with much less carbon.
Reading
- “How the World Really Works by Vaclav Smil | Penguin Random House Canada.” Accessed April 24, 2025. https://www.penguinrandomhouse.ca/books/666342/how-the-world-really-works-by-vaclav-smil/9780593553176.
- SteelWatch. “SteelWatch Explainer: Why Steelmaking Drives Climate Change – and Why It Doesn’t Have to Be This Way.” SteelWatch, January 22, 2025. https://steelwatch.org/steelwatch-explainers/climate/.
- IEA. “Aviation.” Accessed April 24, 2025. https://www.iea.org/energy-system/transport/aviation.
- “Hand Tool | Types & Facts | Britannica.” Accessed April 22, 2025. https://www.britannica.com/technology/hand-tool.
- “Hand Tool - Early Metals, Smelting | Britannica.” Accessed April 25, 2025. https://www.britannica.com/technology/hand-tool/Early-metals-and-smelting.
- He, Jeremy. “21 Chemical Elements and Effects on Steel Mechanical Properties.” Otai Special Steel (blog), October 7, 2015. https://www.astmsteel.com/steel-knowledge/chemical-elements-and-effects-mechanical-properties/.
- Rahulanand. “The Legacy of Puddled Iron in Contemporary Metallurgy.” Jupiter Science (blog), April 1, 2024. https://jupiterscience.com/chemistry/the-legacy-of-puddled-iron-in-contemporary-metallurgy/.
- Sil, Vaclav, op cit, Chapter 3
- “Basic Oxygen Furnace Steelmaking,” January 25, 2024. https://www.steel-technology.com/articles/oxygenfurnace.
- worldsteel.org. “World Steel in Figures 2024.” Accessed April 26, 2025. https://worldsteel.org/data/world-steel-in-figures/world-steel-in-figures-2024/.
- US EPA, OAR. “Greenhouse Gas Equivalencies Calculator.” Data and Tools, August 28, 2015. https://www.epa.gov/energy/greenhouse-gas-equivalencies-calculator.
- “Steelmaking CO2 Emissions by Process Step.” Accessed April 27, 2025. https://www.steelonthenet.com/kb/co2-emissions.html.
- worldsteel.org. “World Steel in Figures 2024.”, op. Cit.
- Steelonthenet.com. “Top 10 Steel Industry Challenges & Strategic Issues.” SteelOnTheNet.com. Accessed April 28, 2025. https://www.steelonthenet.com/steel-challenges.html.